Dear, The students of PGBI 2010
These are some question to be answered. They are:
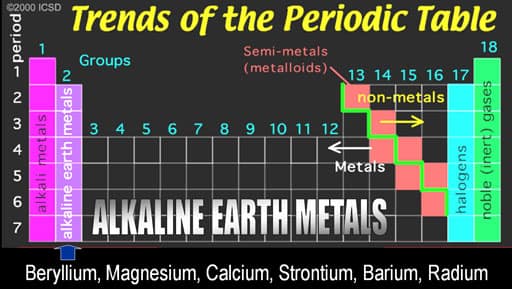
- If one piece of metal, such as Ca or Mg, reacts with atmospheric oxygen, would you expect the product to have a greater or lesser mass than the reacting metal? Why?
- How should you dispose of the products of the reaction of calcium and water?
- What are some of the hazards of working with metals that are as active as the alkaline earth metals? What precautions should you follow?
Okay… Just answer all of the questions above in column of comments below. Don’t forget to put your name before answering that. Please, put the source of your answer. Okay!!!




5 Responses
mbak rahmah emang jago bahasa inggris deh ,,, jadi iri … 😀
@dycko,
Hehehehe biasa aja mas dycko 😀
1. If one piece of metal, such as Ca or Mg, reacts with atmospheric oxygen, it will produce CaO or MgO. And the greater mass of the product CaO, it because Ca is more reactive than Mg. And I expect the greater mass of product because adding oxygen to the metal and making it larger.
2. To dispose the product of the reaction of calcium and water, first, neutralize it, to lower the pH and make it safe to release. Or it can neutralize with a base and then we can dispose them by pouring them into the sink.
3. the hazard of working with metals that are as active as the alkaline earth are fire hazard; it can explode and produce flammable gas if reacts with water. Health hazard; inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solution on contact with water, fire will produce irritating, toxic gasses. Run off from fire control may cause pollution. And the precaution should we follow are; wear safety goggles and laboratory apron at all times during working with the alkaline metal. Avoid touching the alkaline metal directly. Any contact on the skin should be washed with plenty of water. If the exposed area of the skin feels slippery, continue to rinse it with water. Make sure no open flamer are in laboratory when reacting some alkaline metal with water because the gas produce is explosive.
1. By heating or react with Magnesium, oxygen can produce Oxide compound. Magnesium oxide is formed will be a protective layer on metal surfaces.
2Mg (s) + O2 (g) → 2MgO (s)
When react in the air Magnesium with limited oxygen at high temperatures will be able to produce Magnesium Nitride (Mg3N2).
4mg (s) + ½ O2 (g) + N2 (g) → MgO (s) + Mg3N2 (s)
When Mg3N2 reacted with water it will get NH3 gas.
Mg3N2 (s) + 6H2O (l) → 3Mg (OH) 2 (s) + 2NH3 (g).
-If the Ca reacts with oxygen, calcium will reacted, because of its reactivity to form an oxide.
2Ca (s) + O2 (g) -> 2CaO (s)
And the greater mass of the product CaO, it because Ca has large relative atomic mass, 40 gram/mol than Mg,24 gram/mol.So, when we react with oxygen it will produce larger product, as we know that to calculate the mass it be affected with relativity atomic mass.
2. If we react Calsium with water, it will produce Ca(OH)2.
The chemical equation is Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
Calcium hydroxide is known as slaked lime. It is sparingly soluble in water and the resulting mildly alkaline solution is known as lime water which is used to test for the acidic gas carbon dioxide.
Calcium hydroxide solution (Ca(OH)2) is used to detect the presence of carbon dioxide by being bubbled through a solution. It turns cloudy where CO2 is present.
And To dispose the product of the reaction of calcium and water.
We can react it with acid, to get a lower pH and the solution become neutral.
3. the hazard of working with metals that are as active as the alkaline earth are fire hazard; it can explode and produce flammable gas if reacts with water but Metals in the second class are slightly less active. They don’t react with water at room temperature, but they react rapidly with acids.For health;It is extremely dangerous if inhaled. Its effectiveness depends on the content presented and the duration of exposure. If the content of it in air is very high (greater than 1000 mg / m³), an acute condition may occur. This situation resembles pneumonia and is called acute beryllium disease. Determination of community and workplace air effective in avoiding damage to the lungs are most acute.
The precaution should we follow are eliminate all ignition sources (no smoking, flares, sparks or flames in immediate area). Do not touch or walk through spilled material. Stop leak if you can do it without risk. Use water spray to reduce vapors or divert vapor cloud drift. Avoid allowing water runoff to contact spilled material. do not get water on spilled substance or inside containers.Wear safety goggles and laboratory apron at all times during working with the alkaline metal. Loose clothing or long hair should not be allowed around working with reactive metals.
1. 2Mg (s) + O2 (g) → 2MgO (s)
is a reaction of magnesium with oxygen,while calcium react with oxygen, the produc :
2Ca (s) + O2 (g) -> 2CaO (s).
The greater mass of the product CaO, it because Ca is more reactive than Mg. the greater mass is CaO, cause Calcium more reactive rater than magnesium
2. How should you dispose of the products of the reaction of calcium and water?
First neutralize it, to lower the pH and make it safe to release and You can neutralize it with a base
3. if we work with an alkaline soil, the danger that might happen:
Fire and Health Hazard
fire hazard
Excerpt from GUIDE 138 [Substances – Water-Reactive (Emitting Flammable Gases)]:
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard. (ERG, 2008)
healt hazard
Excerpt from GUIDE 138 [Substances – Water-Reactive (Emitting Flammable Gases)]:
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution. (ERG, 2008)
What precautions should you follow?
DO NOT USE WATER OR FOAM.
SMALL FIRE: Dry chemical, soda ash, lime or sand.
LARGE FIRE: DRY sand, dry chemical, soda ash or lime or withdraw from area and let fire burn. Move containers from fire area if you can do it without risk.
FIRE INVOLVING METALS OR POWDERS (ALUMINUM, LITHIUM, MAGNESIUM, ETC.): Use dry chemical, DRY sand, sodium chloride powder, graphite powder or Met-L-X® powder; in addition, for Lithium you may use Lith-X® powder or copper powder. Also, see GUIDE 170.
FIRE INVOLVING TANKS OR CAR/TRAILER LOADS: Fight fire from maximum distance or use unmanned hose holders or monitor nozzles. Do not get water inside containers. Cool containers with flooding quantities of water until well after fire is out. Withdraw immediately in case of rising sound from venting safety devices or discoloration of tank. ALWAYS stay away from tanks engulfed in fire. (ERG, 2008)